Definition:-
In physics, energy is a property of objects which can be transferred to other objects or converted into differentforms, but cannot be created or destroyed.[1]The "ability of a system to perform work" is a common description, but it is difficult to give one single comprehensive definition of energy because of its many forms.[2] For instance, in SI units, energy is measured in joules, and one joule is defined "mechanically", being the energy transferred to an object by the mechanical work of moving it a distance of 1 metre against a force of 1 newton.[note 1] However, there are many other definitions of energy, depending on the context, such as thermal energy, radiant energy, electromagnetic, nuclear, etc., where definitions are derived that are the most convenient.
Common energy forms include the kinetic energy of a moving object, the radiant energy carried by light, thepotential energy stored by an object's position in a force field (gravitational, electric or magnetic), elastic energystored by stretching solid objects, chemical energy released when a fuel burns, and the thermal energy due to an object's temperature. All of the many forms of energy are convertible to other kinds of energy, and obey the law ofconservation of energy which says that energy can be neither created nor be destroyed; however, it can change from one form to another.
For "closed systems" with no external source or sink of energy, the first law of thermodynamics states that a system's energy is constant unless energy is transferred in or out by mechanical work or heat, and that no energy is lost in transfer. This means that it is impossible to create or destroy energy. The second law of thermodynamicsstates that all systems doing work always lose some energy as waste heat. This creates a limit to the amount of energy that can do work by a heating process, a limit called the available energy. Mechanical and other forms of energy can be transformed in the other direction into thermal energy without such limitations.[3] The total energy of a system can be calculated by adding up all forms of energy in the system.
Examples of energy transformation include generating electric energy from heat energy via a steam turbine, or lifting an object against gravity using electrical energy driving a crane motor. Lifting against gravity performs mechanical work on the object and stores gravitational potential energy In the object. If the object falls to ground, gravity does mechanical work on the object which transforms the potential energy in the gravitational field to the kinetic energy released as heat on impact with the ground. Our Sun transforms nuclear potential energy to other forms of energy; its total mass does not decrease due to that in itself (since it still contains the same total energy even if in different forms), but its mass does decrease when the energy escapes out to its surroundings, largely asradiant energy.
Mass and energy are closely related. According to the theory of mass–energy equivalence, any object that has mass when stationary in a frame of reference (called rest mass) also has an equivalent amount of energy whose form is called rest energy in that frame, and any additional energy acquired by the object above that rest energy will increase an object's mass. For example, if you had a sensitive enough scale, you could measure an increase in mass after heating an object.
Living organisms require available energy to stay alive, such as the energy humans get from food. Civilisation gets the energy it needs from energy resources such as fossil fuels. The processes of Earth's climate andecosystem are driven by the radiant energy Earth receives from the sun and the geothermal energy contained within the earth.
Conservation of energy and mass in transformation:-
Energy gives rise to weight when it is trapped in a system with zero momentum, where it can be weighed. It is also equivalent to mass, and this mass is always associated with it. Mass is also equivalent to a certain amount of energy, and likewise always appears associated with it, as described in mass-energy equivalence. The formulaE = mc², derived by Albert Einstein (1905) quantifies the relationship between rest-mass and rest-energy within the concept of special relativity. In different theoretical frameworks, similar formulas were derived by J. J. Thomson (1881), Henri Poincaré (1900), Friedrich Hasenöhrl (1904) and others (see Mass-energy equivalence#History for further information).
Matter may be converted to energy (and vice versa), but mass cannot ever be destroyed; rather, mass/energy equivalence remains a constant for both the matter and the energy, during any process when they are converted into each other. However, since  is extremely large relative to ordinary human scales, the conversion of ordinary amount of matter (for example, 1 kg) to other forms of energy (such as heat, light, and other radiation) can liberate tremendous amounts of energy (~
is extremely large relative to ordinary human scales, the conversion of ordinary amount of matter (for example, 1 kg) to other forms of energy (such as heat, light, and other radiation) can liberate tremendous amounts of energy (~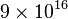 joules = 21 megatons of TNT), as can be seen in nuclear reactors and nuclear weapons. Conversely, the mass equivalent of a unit of energy is minuscule, which is why a loss of energy (loss of mass) from most systems is difficult to measure by weight, unless the energy loss is very large. Examples of energy transformation into matter (i.e., kinetic energy into particles with rest mass) are found in high-energy nuclear physics.
joules = 21 megatons of TNT), as can be seen in nuclear reactors and nuclear weapons. Conversely, the mass equivalent of a unit of energy is minuscule, which is why a loss of energy (loss of mass) from most systems is difficult to measure by weight, unless the energy loss is very large. Examples of energy transformation into matter (i.e., kinetic energy into particles with rest mass) are found in high-energy nuclear physics.
 is extremely large relative to ordinary human scales, the conversion of ordinary amount of matter (for example, 1 kg) to other forms of energy (such as heat, light, and other radiation) can liberate tremendous amounts of energy (~
is extremely large relative to ordinary human scales, the conversion of ordinary amount of matter (for example, 1 kg) to other forms of energy (such as heat, light, and other radiation) can liberate tremendous amounts of energy (~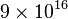 joules = 21 megatons of TNT), as can be seen in nuclear reactors and nuclear weapons. Conversely, the mass equivalent of a unit of energy is minuscule, which is why a loss of energy (loss of mass) from most systems is difficult to measure by weight, unless the energy loss is very large. Examples of energy transformation into matter (i.e., kinetic energy into particles with rest mass) are found in high-energy nuclear physics.
joules = 21 megatons of TNT), as can be seen in nuclear reactors and nuclear weapons. Conversely, the mass equivalent of a unit of energy is minuscule, which is why a loss of energy (loss of mass) from most systems is difficult to measure by weight, unless the energy loss is very large. Examples of energy transformation into matter (i.e., kinetic energy into particles with rest mass) are found in high-energy nuclear physics.
कोई टिप्पणी नहीं:
एक टिप्पणी भेजें