Branches of physics 
Physics deals with the combination of matter and energy. It also deals with a wide variety of systems, about which theories have been developed that are used by physicists. In general, theories are experimentally tested numerous times before they are accepted as correct as a description of Nature (within a certain domain of validity). For instance, the theory of classical mechanics accurately describes the motion of objects, provided they are much larger than atoms and moving at much less than the speed of light. These theories continue to be areas of active research: for instance, a remarkable aspect of classical mechanics known as chaos was discovered in the 20th century, three centuries after the original formulation of classical mechanics by Isaac Newton (1642–1727). These "central theories" are important tools for research in more specialized topics, and any physicist, regardless of his or her specialization, is expected to be literate in them.
Classical mechanics:-
Classical mechanics is a model of the physics of forces acting upon bodies. It is often referred to as "Newtonian mechanics" after Isaac Newton and his laws of motion. It also includes classical approach as given by Hamiltonian and Lagrange methods.
Thermodynamics and statistical mechanics:-
The first chapter of The Feynman Lectures on Physics is about the existence of atoms, which Feynman considered to be the most compact statement of physics, from which science could easily result even if all other knowledge was lost.[1] By modeling matter as collections of hard spheres, it is possible to describe the kinetic theory of gases, upon which classical thermodynamics is based.
Thermodynamics studies the effects of changes in temperature, pressure, and volume on physical systems on themacroscopic scale, and the transfer of energy as heat.[2][3] Historically, thermodynamics developed out of the desire to increase the efficiency of early steam engines.[4]
The starting point for most thermodynamic considerations is the laws of thermodynamics, which postulate thatenergy can be exchanged between physical systems as heat or work.[5] They also postulate the existence of a quantity named entropy, which can be defined for any system.[6] In thermodynamics, interactions between large ensembles of objects are studied and categorized. Central to this are the concepts of system and surroundings. A system is composed of particles, whose average motions define its properties, which in turn are related to one another through equations of state. Properties can be combined to express internal energy and thermodynamic potentials, which are useful for determining conditions for equilibrium and spontaneous processes.
Quantum mechanics
Quantum mechanics is the branch of physics treating atomic andsubatomic systems and their interaction with radiation. It is based on the observation that all forms of energy are released in discrete units or bundles called "quanta". Remarkably, quantum theory typically permits only probable or statistical calculation of the observed features of subatomic particles, understood in terms of wave functions. TheSchrödinger equation plays the role in quantum mechanics that Newton's laws and conservation of energy serve in classical mechanics—i.e., it predicts the future behavior of a dynamic system—and is a wave equation that is used to solve for wavefunctions.
For example, the light, or electromagnetic radiation emitted or absorbed by an atom has only certain frequencies (or wavelengths), as can be seen from the line spectrum associated with the chemical element represented by that atom. The quantum theory shows that those frequencies correspond to definite energies of the light quanta, orphotons, and result from the fact that the electrons of the atom can have only certain allowed energy values, or levels; when an electron changes from one allowed level to another, a quantum of energy is emitted or absorbed whose frequency is directly proportional to the energy difference between the two levels. The photoelectric effect further confirmed the quantization of light.
In 1924, Louis de Broglie proposed that not only do light waves sometimes exhibit particle-like properties, but particles may also exhibit wave-like properties. Two different formulations of quantum mechanics were presented following de Broglie's suggestion. The wave mechanics of Erwin Schrödinger (1926) involves the use of a mathematical entity, the wave function, which is related to the probability of finding a particle at a given point in space. The matrix mechanics of Werner Heisenberg (1925) makes no mention of wave functions or similar concepts but was shown to be mathematically equivalent to Schrödinger's theory. A particularly important discovery of the quantum theory is the uncertainty principle, enunciated by Heisenberg in 1927, which places an absolute theoretical limit on the accuracy of certain measurements; as a result, the assumption by earlier scientists that the physical state of a system could be measured exactly and used to predict future states had to be abandoned. Quantum mechanics was combined with the theory of relativity in the formulation of Paul Dirac. Other developments include quantum statistics, quantum electrodynamics, concerned with interactions between charged particles and electromagnetic fields; and its generalization, quantum field theory.


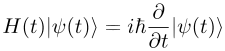
कोई टिप्पणी नहीं:
एक टिप्पणी भेजें